
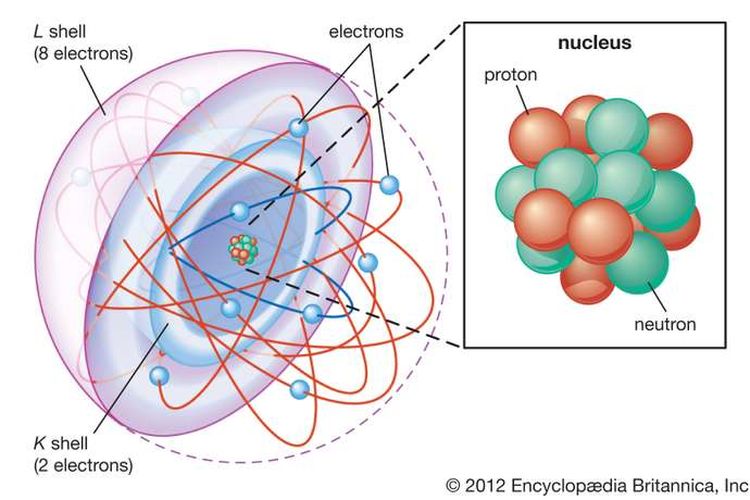
The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. Atomic mass unit (u) is used for expressing the mass of atoms or molecules. The cookie is used to store the user consent for the cookies in the category "Performance". This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. 1 mole H 2 O will contain 3N A atoms 3 × 6. This isotope of carbon has 6 protons and 6 neutrons. Now, 1 molecule of H 2 O contain 3 atoms. carbon, 12C, that is taken as a standard element with an atomic mass of 12. Solution: The 18 g of H 2 O 1 mole of H 2 O N A molecules. Example 3: Calculate the number of atoms present in 18g of H 2 O. The cookies is used to store the user consent for the cookies in the category "Necessary". It means that one molecule of H 2 SO 4 is 98 times as heavy as 1/12 the mass of a C-12 atom. The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional".

The cookie is used to store the user consent for the cookies in the category "Analytics". These cookies ensure basic functionalities and security features of the website, anonymously. To calculate the mass of a single atom of carbon, we just need to divide the molar mass of 12.0 g (0,012 kg) by the number of particles per mole (Avogadros. Necessary cookies are absolutely essential for the website to function properly. Average masses are generally expressed in unified atomic mass units (u), where 1 u is equal to exactly one-twelfth the mass of a neutral atom of carbon-12. The average atomic mass (sometimes called atomic weight) of an element is the weighted average mass of the atoms in a naturally occurring sample of the element. What is the mass exactly equal to 1 12th of the mass of one C 12 atom known as? Then you have 9893 atoms of 12C and 107 atoms of 13C. What is the mass of 3 moles of NaOH Now use this to convert 120 g to moles. Hence, mass of 3 mole of neon 3 × 20 60 gram. What is the mass of 3 moles of neon We know, molar mass of neon ( mass of one mole of neon) 20 gram. Assume that you have, say, 10 000 atoms of carbon. What is the mass of 1 atom of C 12 in grams 1.989×1023 gm. However C-12 will have 6 neutrons whereas C-13 will have 7 neutrons. To find the average atomic mass, you take a certain number of atoms, find the total mass of each isotope, and then divide the total mass of all the atoms by the total number of atoms. How is a c12 and c13 atom alike Both C-12 and C-13 are isotopes and have 6 protons and 6 electrons each. How do you calculate the average atomic mass of carbon isotopes?
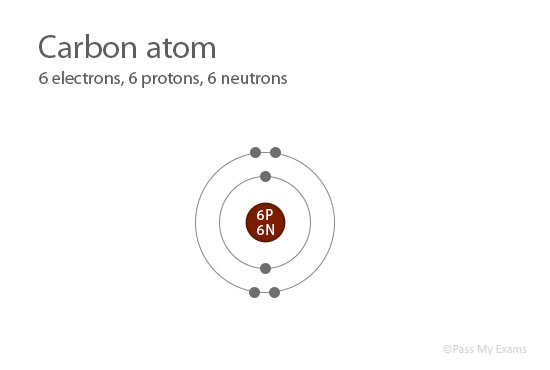
For example, a carbon-12 atom has 6 protons and 6 neutrons, and so has a relative atomic mass of 12. You can always find the relative mass of an element by adding the number of protons to the number of neutrons for the specific isotope of the element you’re considering. How do you calculate the relative atomic mass of carbon-12? READ: How many days can you be on a liquid diet?


 0 kommentar(er)
0 kommentar(er)
